(The main page on transport is here.)
Annex 1: Belgian competent authorities for the transport of dangerous goods
SPF Mobilité et Transports / FOD Mobiliteit en Vervoer
City Atrium
56 Rue du progrès / Vooruitgangstraat 56
1210 Brussels
1) ADR – multimodal aspects (transport by road)
Direction Générale Politique de Mobilité Durable et Ferroviaire
Direction Multimodalité
Marchandises Dangereuses
Tél : 02/277 39 04-05
Mail : MarchandisesDangereuses@mobilit.fgov.be
2) RID (transport by rail)
Service de Sécurité et d’Interopérabilité des Chemins de Fer
Mme Caroline Bailleux
Tél : 02/277 39 16
Mail : caroline.bailleux@mobilit.fgov.be
3) IMDG (transport by boat)
Direction Générale Transport Maritime
M. Patrick Van Lancker
Tél : 059/56 14 62
Mail : Hazmat.Mar@mobilit.fgov.be
4) ICAO TI (transport by air)
Direction Générale Transport Aérien
Direction Opérations
M. Remko Dardenne
Tél : 02/277 43 67
Mail : bcaa.dangerousgoods@mobilit.fgov.be
Annex 2: Belgian regional authorities for ADR
Wallonia
e-mail: adr.adn@spw.wallonie.be
phone: 081/33 66 60 fax: 081/33 65 44
website: spw.wallonie.be
address: SPW Wallonie DRIGM – ADR/ADN - 15 Avenue Prince de Liège, 5100 Namur
Brussels-Capital region
e-mail: bruxellesmobilite@sprb.irisnet.be
phone: 0800 94 001
website: www.bruxellesmobilite.irisnet.be
address: Bruxelles Mobilité, CCN, Rue du Progrès 80, 1030 BRUXELLES
Flemish region
Website : http://www.mobielvlaanderen.be/contactpunt
Address : Vlaamse Overheid, Departement Mobiliteit en Openbare Werken, Afdeling Beleid Mobiliteit en Verkeersveiligheid, Koning Albert II-laan 20 bus 2, Graaf de Ferrarisgebouw, 1000 Brussel
Annex 3: Class 6 - Division 6.2 ("Infectious Substances")
This class comprises substances known to contain or reasonably expected to contain viable micro-organisms, including bacteria, viruses, rickettsia, parasites, fungi, also as recombinant, hybrid or mutant micro-organisms, that are known or reasonably believed to cause disease in animals or humans. They are capable of spreading diseases to humans or animals when exposure to them occurs.
Annex 4: Class 9 ("Miscellaneous Dangerous Substances ")
This Class covers substances and articles which, during carriage, present a danger not covered by the headings of other classes.
Genetically modified micro-organisms which are not dangerous for animals or humans, but which could modify animals, plants, microbiological substances and ecosystems in a way that does not occur naturally, are included in this Class.
Genetically modified micro-organisms which have been approved for deliberate release into the environment (under Part C of Directive 2001/18/EC), are not subject to the provisions of this Class.
Annex 5: P620 (ADR) or PI 620 (IATA) – applies to the transport of Category A (UN 2814 and UN 2900)
P620 or PI 620 requirements consists of:
1) a triple packaging comprising of:
- leakproof primary receptacle(s);
- a leakproof secondary packaging;
- a rigid outer packaging.
Sufficient absorbent material is placed between the primary receptacle(s) and the secondary packaging to absorb all fluid in case of breakage or leakage. If multiple primary receptacles are send, they must be wrapped individually to prevent contact between them.
2) an itemized list of contents that is enclosed between the secondary packaging and the outer packaging.
There is no maximum quantity per package for surface transport. For air transport the quantity is limited to :
- 50 ml or 50 g per package for passenger aircraft,
- 4 litres or 4 kg per package for cargo aircraft.
3) Technical characteristics:
The primary receptacle or the secondary packaging shall support a pressure differential of not less than 95 kPa and temperatures in the range -40 °C to +55 °C.
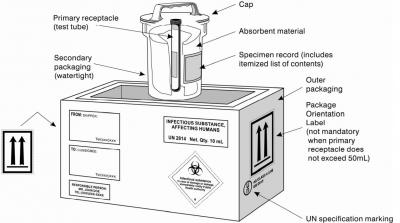
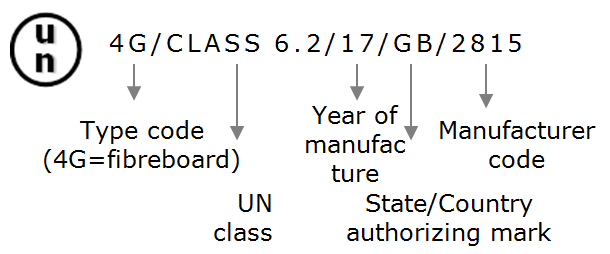
Packaging for Category B infectious substances must be UN-approved after successfully having passed several performance tests: drop test from a height of 9 m, puncture test with the fall of a steel rod on the package from a height of 1 m, stacking test (withstanding to a force applied to the top surface during 24 h) and internal pressure test. The tests are performed by packaging suppliers approved by national authorities, thus UN packaging symbol and specification marking for Category A must appear on the outer packaging, for example:
Example of packaging complying with PI 620 (click for lager):
(from: WHO Guidance on regulations for the transport of infectious substances 2015–2016)
4) Specific requirements:
- Substances shipped at ambient or higher temperatures:
Primary receptacles must be of glass, metal or plastic. These receptacles should be sealed to ensure leakproof (e.g. by heat seal, skirted stopper or metal crimp seal). If screw caps are used, these must be secured by positive means (e.g. tape, paraffin).
- Substances shipped refrigerated or frozen:
- Ice, dry ice or other refrigerant must be placed around the secondary packaging(s) or in an overpack if any.
- Interior support must be provided to secure the secondary packaging(s) in the original position after the ice or dry ice has dissipated.
- If ice is used, the outer packaging or overpack must be leakproof.
- If dry ice is used, the outer packaging or overpack must permit the release of carbon-dioxide gas.
- The primary receptacle and the secondary packaging must maintain their integrity at the temperature of the refrigerant used.
- Substances consigned in liquid nitrogen:
Plastic primary receptacles and the secondary packaging must be capable of withstanding very low temperatures. Provisions for the consignment of liquid nitrogen must also be fulfilled. The primary receptacle and secondary packaging must maintain their integrity at the temperature of the refrigerant used.
- Lyophilized substances:
Primary receptacles must be sealed (flame-sealed glass ampoules or rubber-stoppered glass vials fitted with metal seals).
Annex 6: P650 (ADR) or PI 650 (IATA) – applies to the transport of Category B (UN 3373)
P650 or PI 650 requirements consists of:
1) a triple packaging comprising of:
- leakproof primary receptacle(s);
- a leakproof secondary packaging;
- a rigid outer packaging.
Primary receptacles must be packed in secondary packaging in such a way that, under normal conditions of transport, they cannot break, be punctured or leak. For liquids, sufficient absorbent material is placed between the primary receptacle(s) and the secondary packaging to absorb the entire contents of the primary receptacle(s) in case of breakage or leakage. The secondary packaging must not be necessary rigid, it could be also, for example, a hermetically sealable plastic bag.
2) an itemized list of contents that is enclosed between the secondary packaging and the outer packaging.
There is no maximum quantity per package for surface transport. For air transport the quantity is limited to :
- 1 litre for liquid substances in one primary receptacle, and 4 litres for the complete packaging.
- 4 kg for solid substances.
3) Technical characteristics: The primary receptacle or the secondary packaging shall support a pressure differential of not less than 95 kPa and temperatures in the range -40 °C to +55 °C.
Packaging for Category B infectious substances must not be UN-certified, so no UN packaging symbol and specification marking are required on the outer packaging. However, the completed package must be successfully pass the drop test from a height of 1,2 m.
Example of packaging complying with PI 650:
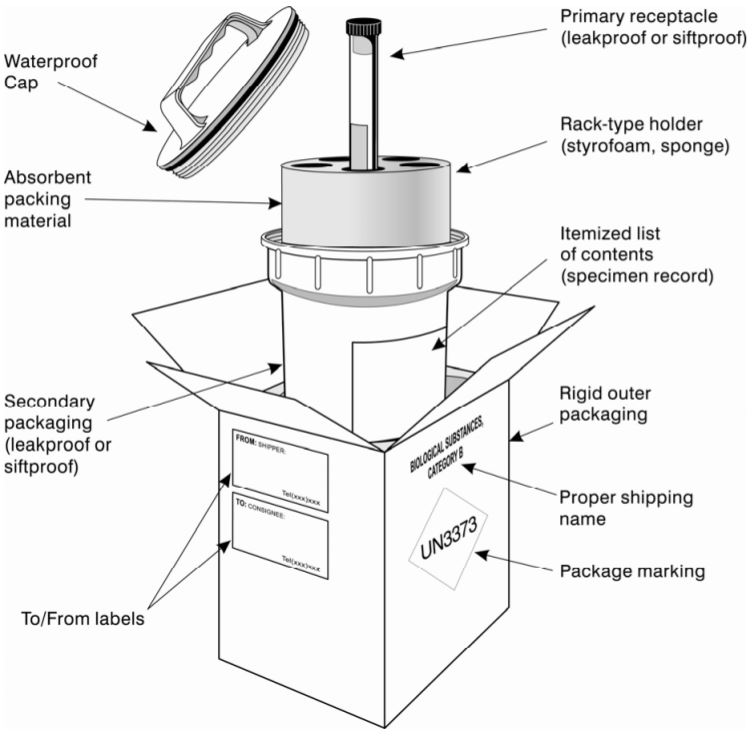
(from: WHO Guidance on regulations for the transport of infectious substances 2015–2016)
4) Specific requirements:
- Substances shipped refrigerated, frozen or in liquid nitrogen:
- Ice, dry ice or other refrigerant must be placed outside the secondary packaging(s) or in an overpack if any.
- Interior support must be provided to secure the secondary packaging(s) in the original position after the ice or dry ice has dissipated.
- If ice is used, the outer packaging or overpack must be leakproof.
- If dry ice is used, the outer packaging or overpack must permit the release of carbon-dioxide gas.
- The primary receptacle and the secondary packaging must maintain their integrity at the temperature of the refrigerant used.
Annex 7: P904 (ADR) or PI 959 (IATA) – applies to the transport of GMOs & GMMs (UN 3245)
Packagings for P904 or PI 959 consist of a triple packaging comprising of:
- leakproof primary receptacle(s);
- a leakproof secondary packaging;
- a rigid outer packaging.
If multiple fragile primary receptacles are placed in a single secondary packaging they must be individually wrapped or separated to prevent contact between them. For liquids, sufficient absorbent material is placed between the primary receptacle(s) and the secondary packaging to absorb the entire contents of the primary receptacle(s) in case of breakage or leakage.
An outer packaging which must be strong enough for its capacity, weight and intended use and must display the UN3245 label as well as the sender and recipient’s full name and address.
There are no UN-certifying requirements for the packaging.
Annex 8: Non-exhaustive list of micro-organisms of Category A
These are indicative examples of infectious substances included in category A in any form unless otherwise indicated.
UN Number and proper shipping name: UN 2814 Infectious substance, affecting humans
- Bacillus anthracis (cultures only)
- Brucella abortus (cultures only)
- Brucella melitensis (cultures only)
- Brucella suis (cultures only)
- Burkholderia mallei - Pseudomonas mallei – Glanders (cultures only)
- Burkholderia pseudomallei – Pseudomonas pseudomallei (cultures only)
- Chlamydia psittaci - avian strains (cultures only)
- Clostridium botulinum (cultures only)
- Coccidioides immitis (cultures only)
- Coxiella burnetii (cultures only)
- Crimean-Congo hemorrhagic fever virus
- Dengue virus (cultures only)
- Eastern equine encephalitis virus (cultures only)
- Escherichia coli, verotoxigenic (cultures only) **
- Ebola virus
- Flexal virus
- Francisella tularensis (cultures only)
- Guanarito virus
- Hantaan virus
- Hantaviruses causing haemorrhagic fever with renal syndrome
- Hendra virus
- Hepatitis B virus (cultures only)
- Herpes B virus (cultures only)
- Human immunodeficiency virus (cultures only)
- Highly pathogenic avian influenza virus (cultures only)
- Japanese Encephalitis virus (cultures only)
- Junin virus
- Kyasanur Forest disease virus
- Lassa virus
- Machupo virus
- Marburg virus
- Monkeypox virus
- Mycobacterium tuberculosis (cultures only) **
- Nipah virus
- Omsk hemorrhagic fever virus
- Poliovirus (cultures only)
- Rabies virus (cultures only)
- Rickettsia prowazekii (cultures only)
- Rickettsia rickettsii (cultures only)
- Rift Valley fever virus (cultures only)
- Russian spring-summer encephalitis virus (cultures only)
- Sabia virus
- Shigella dysenteriae type 1 (cultures only) **
- Tick-borne encephalitis virus (cultures only)
- Variola virus
- Venezuelan equine encephalitis virus (cultures only)
- West Nile virus (cultures only)
- Yellow fever virus (cultures only)
- Yersinia pestis (cultures only)
UN Number and proper shipping name: UN 2900 Infectious substance, affecting animals only
- African swine fever virus (cultures only)
- Avian paramyxovirus Type 1 – velogenic Newcastle disease virus (cultures only)
- Bluetongue virus *
- Classical swine fever virus (cultures only)
- Foot and mouth disease virus (cultures only)
- Lumpy skin disease virus (cultures only)
- Mycoplasma mycoides - Contagious bovine pleuropneumonia (cultures only)
- Peste des petits ruminants virus (cultures only)
- Rinderpest virus (cultures only)
- Sheep-pox virus (cultures only)
- Goatpox virus (cultures only)
- Swine vesicular disease virus (cultures only)
- Vesicular stomatitis virus (cultures only)
Notes:
* Bluetongue virus is on the indicative list in ADR but not IATA. However, since all shipments involve road transport it should always be classified and transported as a category A infectious substance.
** For surface transport (ADR) nevertheless, when the cultures are intended for diagnostic or clinical purposes, they may be classified as infectious substances of Category B.